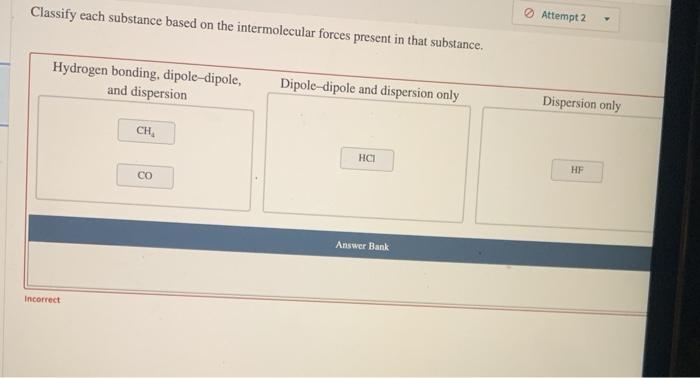
Dive into the fascinating world of ionic bonds with our comprehensive Ionic Bonds Gizmos Answer Key. This guide unlocks the secrets of ionic compound formation, properties, and applications, providing a clear understanding of these essential chemical entities.
As we delve deeper into the topic, you’ll discover the intricacies of ionic bond formation, explore the unique characteristics of ionic compounds, and uncover their diverse applications in various industries.
Definition of Ionic Bonds
Ionic bonds are formed when one atom transfers one or more electrons to another atom, resulting in the formation of positively and negatively charged ions. These oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.Ionic
bonds are typically formed between a metal and a non-metal. The metal atom loses one or more electrons to achieve a stable electron configuration, becoming a positively charged ion (cation). The non-metal atom gains these electrons, becoming a negatively charged ion (anion).
The attraction between the cation and the anion forms the ionic bond.
Characteristics of Ionic Bonds
Ionic bonds are characterized by several key features:
- Strong electrostatic attraction:The oppositely charged ions are strongly attracted to each other, resulting in the formation of stable compounds.
- High melting and boiling points:Ionic compounds have high melting and boiling points due to the strong electrostatic forces holding the ions together.
- Solubility in water:Ionic compounds are generally soluble in water because water molecules can surround and separate the ions, forming hydrated ions.
- Electrical conductivity:Ionic compounds conduct electricity when dissolved in water or melted because the ions can move freely and carry an electrical charge.
Formation of Ionic Bonds
Ionic bonds form when one atom transfers one or more electrons to another atom, creating oppositely charged ions that attract each other. This process typically occurs between a metal and a non-metal.
The metal atom loses electrons, forming a positively charged cation. The non-metal atom gains electrons, forming a negatively charged anion. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Electron Transfer
The number of electrons transferred depends on the valence electrons of the atoms involved. Valence electrons are the electrons in the outermost energy level of an atom, and they determine the chemical properties of the atom.
Metals tend to have few valence electrons, while non-metals tend to have many valence electrons. When a metal atom reacts with a non-metal atom, the metal atom will typically transfer one or more valence electrons to the non-metal atom.
Examples of Ionic Bond Formation, Ionic bonds gizmos answer key
- Sodium (Na) and chlorine (Cl) react to form sodium chloride (NaCl). Sodium has one valence electron, which it transfers to chlorine. Chlorine has seven valence electrons, and it gains one electron from sodium to achieve a stable octet of valence electrons.
- Magnesium (Mg) and oxygen (O) react to form magnesium oxide (MgO). Magnesium has two valence electrons, which it transfers to oxygen. Oxygen has six valence electrons, and it gains two electrons from magnesium to achieve a stable octet of valence electrons.
Properties of Ionic Compounds: Ionic Bonds Gizmos Answer Key

Ionic compounds possess distinctive physical and chemical properties due to the strong electrostatic forces between their constituent ions.
Physical Properties
Ionic compounds typically have high melting and boiling points. The strong electrostatic attraction between ions requires significant energy to overcome, resulting in high melting and boiling temperatures. For example, sodium chloride (NaCl) melts at 801°C and boils at 1413°C.Ionic compounds are generally soluble in polar solvents, such as water.
The polar solvent molecules can surround and solvate the ions, weakening the electrostatic attraction between them and allowing them to dissolve. Conversely, ionic compounds are insoluble in nonpolar solvents, such as hydrocarbons.
Chemical Properties
Ionic compounds are typically unreactive with other ionic compounds due to the strong electrostatic attraction between their ions. However, they can react with substances that can donate or accept electrons, such as metals or nonmetals. For example, sodium (Na) reacts with chlorine (Cl) to form sodium chloride (NaCl), while calcium oxide (CaO) reacts with water to form calcium hydroxide (Ca(OH) 2).Ionic
compounds are good conductors of electricity when dissolved in water or melted. In these states, the ions are free to move and carry electric current. However, ionic compounds are poor conductors of electricity in the solid state because the ions are fixed in place by the strong electrostatic attraction between them.
Applications of Ionic Compounds
Ionic compounds are widely used in various industries due to their unique properties. Their applications range from everyday products to advanced technologies.
One major application of ionic compounds is in the production of ceramics. Ceramics are made by heating inorganic, non-metallic materials, such as clay, at high temperatures. The resulting materials are hard, durable, and resistant to heat and chemicals. Ceramics are used in a variety of products, including tiles, pottery, and cookware.
In Medicine
Ionic compounds are also essential in the medical field. For instance, sodium chloride (NaCl), commonly known as table salt, is used to replenish electrolytes in the body. Calcium carbonate (CaCO3) is used as an antacid to neutralize stomach acid. Additionally, iodine (I-) is used in disinfectants and as a contrast agent in medical imaging.
Understanding ionic bonds gizmos answer key can be a breeze with the right resources. And if you’re looking to delve into US history, don’t miss out on the gateway to us history answers . This comprehensive guide will help you navigate through key historical events and figures with ease.
With these resources, you’ll be well-equipped to master both ionic bonds and American history.
In Batteries
Ionic compounds play a crucial role in the functioning of batteries. Lithium-ion batteries, commonly found in electronic devices, utilize ionic compounds as electrolytes to facilitate the flow of ions between the electrodes. These batteries provide long-lasting power and are rechargeable, making them suitable for portable devices.
In Water Treatment
Ionic compounds are employed in water treatment processes. Aluminum sulfate (Al2(SO4)3) is used as a coagulant to remove impurities and clarify water. Sodium hypochlorite (NaClO) is used as a disinfectant to kill bacteria and other microorganisms.
Gizmos Answer Key
The Gizmos answer key provides detailed explanations and step-by-step solutions to the interactive simulations and activities in the Ionic Bonds Gizmo. This table summarizes the key questions, answers, explanations, and direct links to the corresponding Gizmos simulations for easy reference.
Gizmo Answer Key Table
The following table presents the Gizmos answer key in a structured and easy-to-navigate format:
| Question | Answer | Explanation | Gizmo Simulation Link |
|---|---|---|---|
| What is an ionic bond? | An ionic bond is a chemical bond formed between two oppositely charged ions. | An ionic bond is formed when one atom transfers one or more electrons to another atom, creating two oppositely charged ions. The positive ion is called a cation, and the negative ion is called an anion. The electrostatic attraction between the cation and the anion holds the ionic bond together. | Ionic Bonds Gizmo |
| How are ionic bonds formed? | Ionic bonds are formed when a metal atom loses one or more electrons to a nonmetal atom. | The metal atom becomes a positively charged cation, and the nonmetal atom becomes a negatively charged anion. The electrostatic attraction between the cation and the anion holds the ionic bond together. | Ionic Bonds Gizmo |
| What are the properties of ionic compounds? | Ionic compounds are typically solids at room temperature, have high melting and boiling points, are soluble in water, and conduct electricity when dissolved in water or melted. | The strong electrostatic attraction between the ions in an ionic compound holds the compound together in a solid state. The high melting and boiling points are due to the strong interionic forces. The solubility of ionic compounds in water is due to the polar nature of water molecules, which can solvate the ions. The electrical conductivity of ionic compounds in water or molten state is due to the mobility of the ions. | Ionic Bonds Gizmo |
| What are some applications of ionic compounds? | Ionic compounds are used in a wide variety of applications, including as table salt, fertilizer, and in batteries. | Table salt (NaCl) is an ionic compound used to season food. Fertilizers are ionic compounds that provide essential nutrients to plants. Batteries use ionic compounds as electrolytes to conduct electricity. | Ionic Bonds Gizmo |
Clarifying Questions
What are ionic bonds?
Ionic bonds are chemical bonds formed between atoms when one atom transfers one or more electrons to another atom, creating oppositely charged ions that are attracted to each other.
What are some examples of ionic compounds?
Examples of ionic compounds include sodium chloride (NaCl), potassium iodide (KI), and calcium oxide (CaO).
What are the properties of ionic compounds?
Ionic compounds are typically solids with high melting and boiling points, are soluble in water, and conduct electricity when dissolved or molten.