Chemistry unit 7 reaction equations worksheet 1 – Welcome to Chemistry Unit 7: Reaction Equations Worksheet 1. This worksheet is designed to help you understand the basics of chemical equations, including how to balance them and use them to predict the products of a reaction. We will also explore the different types of chemical reactions and how to identify them from a given chemical equation.
By the end of this worksheet, you will be able to:
- Define chemical equations and explain their importance.
- Explain the concept of balancing chemical equations.
- Provide examples of balanced and unbalanced chemical equations.
- Discuss the different types of chemical reactions.
- Provide examples of each type of reaction.
- Explain how to identify the type of reaction from a given chemical equation.
- Describe the steps involved in balancing chemical equations.
- Explain the concept of coefficients and how they are used to balance equations.
- Provide a step-by-step guide to balancing chemical equations.
- Define stoichiometry and explain its importance in chemistry.
- Discuss the concept of mole ratios and how they are used in stoichiometric calculations.
- Provide examples of stoichiometric calculations, including mass-to-mass, mass-to-volume, and volume-to-volume calculations.
- Discuss the applications of chemical equations in various fields.
Introduction to Chemical Equations
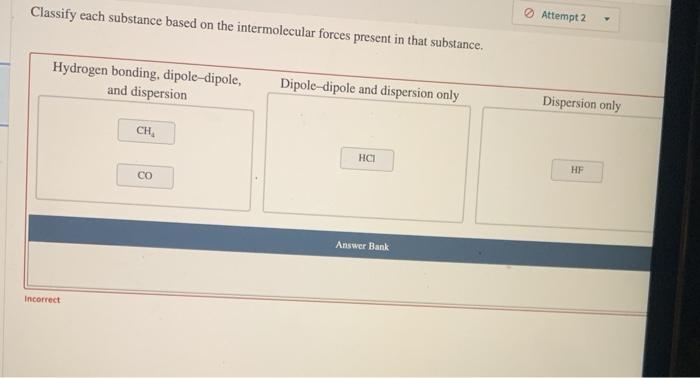
Chemical equations are symbolic representations of chemical reactions. They provide information about the reactants, products, and the stoichiometry of the reaction. Balancing chemical equations is crucial to ensure that the number of atoms of each element is the same on both sides of the equation.
An unbalanced chemical equation is one in which the number of atoms of each element is not the same on both sides of the equation. A balanced chemical equation is one in which the number of atoms of each element is the same on both sides of the equation.
Types of Chemical Reactions: Chemistry Unit 7 Reaction Equations Worksheet 1
Combination Reactions
- Two or more substances combine to form a single product.
- Example: 2Na + Cl 2→ 2NaCl
Decomposition Reactions
- A single compound breaks down into two or more simpler substances.
- Example: 2H 2O → 2H 2+ O 2
Single Displacement Reactions, Chemistry unit 7 reaction equations worksheet 1
- One element replaces another element in a compound.
- Example: Fe + 2HCl → FeCl 2+ H 2
Double Displacement Reactions
- Two compounds exchange ions to form two new compounds.
- Example: NaCl + AgNO 3→ AgCl + NaNO 3
Balancing Chemical Equations
Balancing chemical equations involves adjusting the coefficients in front of each chemical formula to ensure that the number of atoms of each element is the same on both sides of the equation.
Steps for balancing chemical equations:
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Adjust the coefficients in front of each chemical formula to balance the number of atoms of each element.
- Check to make sure that the equation is balanced.
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
Mole ratios are used to convert between the amounts of different substances in a chemical reaction.
Stoichiometric calculations involve using mole ratios to calculate the amount of reactants or products needed or produced in a chemical reaction.
Applications of Chemical Equations
- Predicting the products of a reaction
- Calculating the amount of reactants or products needed
- Understanding chemical processes in everyday life
Key Questions Answered
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction. It shows the chemical formulas of the reactants and products of the reaction, as well as the stoichiometry of the reaction.
What is the importance of balancing chemical equations?
Balancing chemical equations is important because it ensures that the number of atoms of each element is the same on both sides of the equation. This is necessary for the law of conservation of mass to be upheld.
What are the different types of chemical reactions?
There are many different types of chemical reactions, including combination, decomposition, single displacement, and double displacement reactions.
How do I identify the type of reaction from a given chemical equation?
To identify the type of reaction from a given chemical equation, look at the reactants and products. Combination reactions have two or more reactants that combine to form a single product. Decomposition reactions have a single reactant that breaks down into two or more products.
Single displacement reactions have a metal that replaces another metal in a compound. Double displacement reactions have two compounds that exchange ions to form two new compounds.