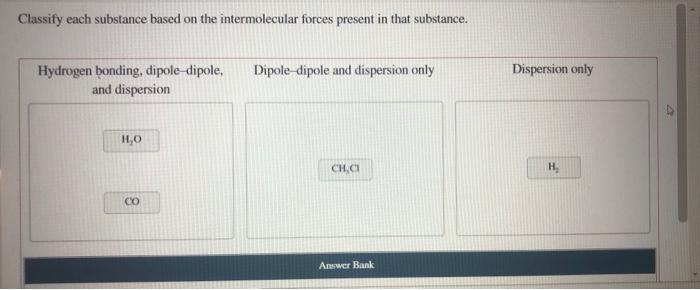
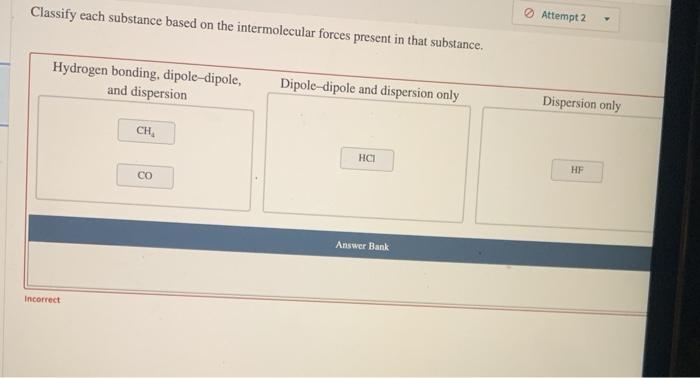
Classify each substance based on the intermolecular forces – Intermolecular forces, the forces that act between molecules, play a crucial role in determining the properties and behavior of substances. Classifying substances based on their intermolecular forces provides a systematic approach to understanding and predicting their physical and chemical characteristics.
This guide explores the different types of intermolecular forces, their influence on substance classification, and their impact on physical properties, chemical reactivity, and practical applications.
Introduction
Intermolecular forces (IMFs) are attractive forces that act between molecules. They determine the physical properties of substances and influence their chemical reactivity. The three main types of IMFs are dipole-dipole interactions, hydrogen bonding, and van der Waals forces.
Classification of Substances
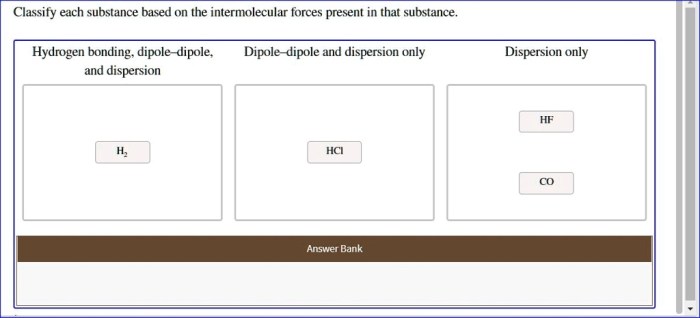
IMFs can be used to classify substances into different types. Ionic compounds are held together by strong electrostatic forces between ions. Molecular compounds are held together by weaker IMFs, such as dipole-dipole interactions and van der Waals forces. Network solids are held together by covalent bonds that form a continuous network throughout the solid.
- Ionic compounds: NaCl, KCl, CaO
- Molecular compounds: H 2O, CH 4, CO 2
- Network solids: diamond, graphite, quartz
Physical Properties
IMFs influence the physical properties of substances. Substances with strong IMFs have higher melting points and boiling points. They are also more viscous and less volatile. Substances with weak IMFs have lower melting points and boiling points. They are also less viscous and more volatile.
| IMF | Melting point | Boiling point | Viscosity | Volatility |
|---|---|---|---|---|
| Dipole-dipole | Moderate | Moderate | Moderate | Moderate |
| Hydrogen bonding | High | High | High | Low |
| van der Waals forces | Low | Low | Low | High |
Chemical Reactivity
IMFs can affect the chemical reactivity of substances. Substances with strong IMFs are less reactive because the molecules are held together more tightly. Substances with weak IMFs are more reactive because the molecules are held together less tightly.
Applications
The classification of substances based on IMFs is used in various fields. In chemistry, it helps us understand and predict the behavior of substances in different reactions. In materials science, it helps us design materials with specific properties. In medicine, it helps us develop drugs that interact with specific targets.
Questions Often Asked: Classify Each Substance Based On The Intermolecular Forces
What are the different types of intermolecular forces?
Intermolecular forces include dipole-dipole interactions, hydrogen bonding, and van der Waals forces.
How do intermolecular forces influence substance classification?
Intermolecular forces determine whether a substance is ionic, molecular, or a network solid.
What is the relationship between intermolecular forces and physical properties?
Intermolecular forces influence melting point, boiling point, and solubility.